Page 78 - International safety guide for oil tankers and terminals
P. 78
INTERNATIONAL SAFETY GUIDE FOR OIL TANKERS AND TERMINALS
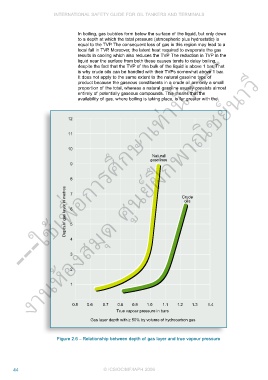
In boiling, gas bubbles form below the surface of the liquid, but only down
to a depth at which the total pressure (atmospheric plus hydrostatic) is
---ใช้เพื่อการศึกษาเท่านั้น---
equal to the TVP. The consequent loss of gas in this region may lead to a
local fall in TVP. Moreover, the latent heat required to evaporate the gas
results in cooling which also reduces the TVP. The reduction in TVP in the
งานห้องสมุด ศูนย์ฝกพาณิชย์นาวี
liquid near the surface from both these causes tends to delay boiling,
despite the fact that the TVP of the bulk of the liquid is above 1 bar. That
is why crude oils can be handled with their TVPs somewhat above 1 bar.
It does not apply to the same extent to the natural gasoline type of
product because the gaseous constituents in a crude oil are only a small
proportion of the total, whereas a natural gasoline usually consists almost
entirely of potentially gaseous compounds. This means that the
availability of gas, where boiling is taking place, is far greater with the
12
11
10
Naturall
gasoline
gasolines
ึ
9
8
Depth of gas layer in metres 6 Crude
7
oils
5
4
3
2
1
0.5 0.6 0.7 0.8 0.9 1.0 1.1 1.2 1.3 1.4
True vapour pressure in bars
Gas layer depth with 50% by volume of hydrocarbon gas
Figure 2.6 – Relationship between depth of gas layer and true vapour pressure
44 © ICS/OCIMF/IAPH 2006