Page 124 - International safety guide for oil tankers and terminals
P. 124
INTERNATIONAL SAFETY GUIDE FOR OIL TANKERS AND TERMINALS
tanks at all times with an atmosphere having an oxygen content of not more
than 8% by volume except when it is necessary for the tank to be gas free.
---ใช้เพื่อการศึกษาเท่านั้น---
When using flue gas from a main or auxiliary boiler, an oxygen level of less
than 5% can generally be obtained, depending on the quality of combustion
SOย์ฝกพาณิชย์นาวี
control and the load on the boiler.
When an independent inert gas generator or a gas turbine plant with
afterburner is fitted, the oxygen content can be automatically controlled within
finer limits, usually within the range 1.5% to 2.5% by volume.
In certain ports, the maximum oxygen content of inert gas in the cargo tanks
may be set at 5% to meet particular safety requirements, such as the
operation of a vapour emission control system. Where such a limitation is in
place, the ship should be advised of the requirements in the pre-arrival
information exchange.
Efficient scrubbing of the inert gas is essential, particularly for the reduction of
the sulphur dioxide content. High levels of sulphur dioxide increase the acidic
characteristic of the inert gas, which is harmful for personnel and may cause
accelerated corrosion to the structure of a ship.
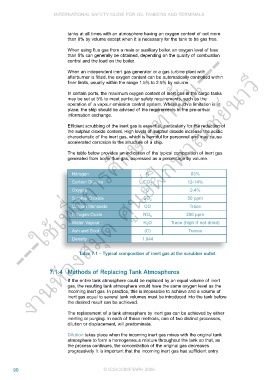
The table below provides an indication of the typical composition of inert gas
generated from boiler flue gas, expressed as a percentage by volume.
ึ
Nitrogen N 83%
งานห้องสมุด ศูน
Carbon Dioxide CO 2 12-14%
Oxygen O 2-4%
Sulphur Dioxide 2 50 ppm
Carbon Monoxide CO Trace
Nitrogen Oxide NO X 200 ppm
Water Vapour H O Trace (high if not dried)
2
Ash and Soot (C) Traces
Density 1.044
Table 7.1 – Typical composition of inert gas at the scrubber outlet
7.1.4 Methods of Replacing Tank Atmospheres
If the entire tank atmosphere could be replaced by an equal volume of inert
gas, the resulting tank atmosphere would have the same oxygen level as the
incoming inert gas. In practice, this is impossible to achieve and a volume of
inert gas equal to several tank volumes must be introduced into the tank before
the desired result can be achieved.
The replacement of a tank atmosphere by inert gas can be achieved by either
inerting or purging. In each of these methods, one of two distinct processes,
dilution or displacement, will predominate.
Dilution takes place when the incoming inert gas mixes with the original tank
atmosphere to form a homogeneous mixture throughout the tank so that, as
the process continues, the concentration of the original gas decreases
progressively. It is important that the incoming inert gas has sufficient entry
90 © ICS/OCIMF/IAPH 2006